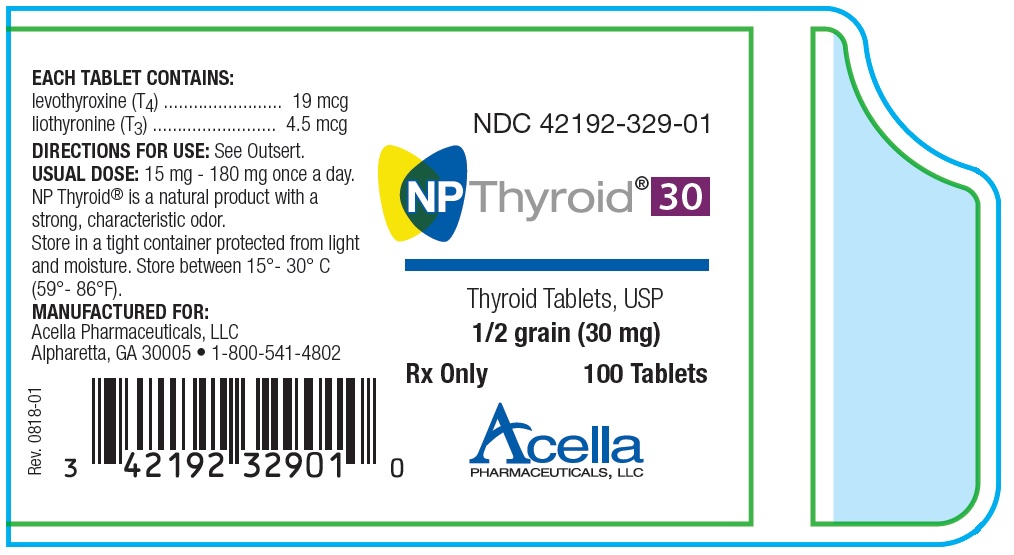
COMPANY ANNOUNCEMENT – Acella Pharmaceuticals, LLC Issues Voluntary Nationwide Recall
May 22, 2020: “Acella Pharmaceuticals, LLC is voluntarily recalling a total of 13 lots of 30-mg, 60-mg and 90-mg NP Thyroid® (thyroid tablets, USP) to the consumer level. The products are being recalled because our testing has found these lots to be superpotent. The product may have up to 115.0% of the labeled amount of Liothyronine (T3).” Continue reading on FDA.gov
The post COMPANY ANNOUNCEMENT – Acella Pharmaceuticals, LLC Issues Voluntary Nationwide Recall appeared first on WAGNER, JONES, KOPFMAN & ARTENIAN.
from
https://wagnerjones.com/company-announcement-acella-pharmaceuticals-llc-issues-voluntary-nationwide-recall/
No comments:
Post a Comment